Wrybill Anarhynchus frontalis Scientific name definitions
- VU Vulnerable
- Names (26)
- Monotypic
Popko Wiersma and Guy M. Kirwan
Version: 1.1 — Published October 24, 2023
Revision Notes
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Кривоклюн дъждосвирец |
| Catalan | corriol bectort |
| Croatian | krivokljuni kulik |
| Czech | kulík křivozobý |
| Dutch | Scheefsnavelplevier |
| English | Wrybill |
| English (United States) | Wrybill |
| Estonian | käändnokk-tüll |
| Finnish | kieronokkatylli |
| French | Pluvier anarhynque |
| French (Canada) | Pluvier anarhynque |
| French (French Guiana) | Gravelot anarhynque |
| German | Schiefschnabel |
| Icelandic | Króklóa |
| Japanese | ハシマガリチドリ |
| Maori | Ngutu Pare | Wrybill |
| Norwegian | skjevnebblo |
| Polish | skrętodziób |
| Russian | Кривоклювый зуёк |
| Serbian | Krivokljuni žalar |
| Slovak | kulík krivozobý |
| Spanish | Chorlitejo Piquituerto |
| Spanish (Spain) | Chorlitejo piquituerto |
| Swedish | snednäbb |
| Turkish | Eğri Gagalı Cılıbıt |
| Ukrainian | Пісочник криводзьобий |
Revision Notes
Leo Gilman prepared the account for the 2023 Clements taxonomy update.
Anarhynchus frontalis Quoy & Gaimard, 1832
PROTONYM:
Anarhynchus frontalis
Quoy & Gaimard, 1832. Voyage de découvertes de la corvette l'Astrolabe, exécuté par ordre du Roi, pendant les années 1826-1827-1828-1829, sous le commandement de M. J. Dumont d'Urville (etc.). Zoologie. Vol. 1 1, p.252.
TYPE LOCALITY:
Bale Chouraki, i.e. Hauraki Gulf, North Island, New Zealand.
SOURCE:
Avibase, 2024
Definitions
- ANARHYNCHUS
- frontale / frontalis
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
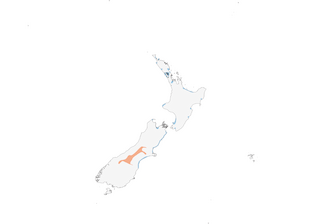
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Wrybill