Pink-footed Goose Anser brachyrhynchus Scientific name definitions
- LC Least Concern
- Names (39)
- Monotypic
Carles Carboneras and Guy M. Kirwan
Version: 1.0 — Published March 4, 2020
Text last updated February 8, 2016
Text last updated February 8, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Asturian | Gansu picucurtiu |
| Basque | Antzara mokolaburra |
| Bulgarian | Късоклюна гъска |
| Catalan | oca de bec curt |
| Croatian | kratkokljuna guska |
| Czech | husa krátkozobá |
| Danish | Kortnæbbet Gås |
| Dutch | Kleine rietgans |
| English | Pink-footed Goose |
| English (United States) | Pink-footed Goose |
| Estonian | lühinokk-hani |
| Faroese | Íslandsgás |
| Finnish | lyhytnokkahanhi |
| French | Oie à bec court |
| French (Canada) | Oie à bec court |
| Galician | Ganso de bico curto |
| German | Kurzschnabelgans |
| Greek | Βραχύραμφη Χήνα |
| Hebrew | אווז קצר-מקור |
| Hungarian | Rövidcsőrű lúd |
| Icelandic | Heiðagæs |
| Italian | Oca zamperosee |
| Japanese | コザクラバシガン |
| Latvian | Īsknābja zoss |
| Lithuanian | Trumpasnapė žąsis |
| Norwegian | kortnebbgås |
| Polish | gęś krótkodzioba |
| Portuguese (Portugal) | Ganso-de-bico-curto |
| Romanian | Gâscă cu cioc scurt |
| Russian | Короткоклювый гуменник |
| Serbian | Kratkokljuna guska |
| Slovak | hus krátkozobá |
| Slovenian | Kratkokljuna gos |
| Spanish | Ánsar Piquicorto |
| Spanish (Mexico) | Ganso Piquicorto |
| Spanish (Spain) | Ánsar piquicorto |
| Swedish | spetsbergsgås |
| Turkish | Küçük Tarla Kazı |
| Ukrainian | Гуменник короткодзьобий |
Anser brachyrhynchus Baillon, 1834
PROTONYM:
Anser Brachyrhynchus
Baillon, 1834. Mémoires de la Société Royale d'Émulation d'Abbeville (1833), p.74.
TYPE LOCALITY:
Abbeville, lower Somme River, France.
SOURCE:
Avibase, 2024
Definitions
- ANSER
- anser
- brachyrhynchus / brachyrrhynchus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
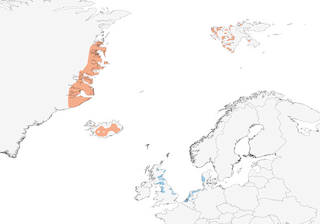
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Pink-footed Goose















































